Laser Cooling of Aluminum Chloride
Ultracold molecules offer a vast number of applications, ranging from searches for novel physics, probes for possible variations of fundamental constants, precision measurements, studies of many-body quantum systems, and manipulation of the outcome of chemical reactions.
In order to realize these applications, an ultracold and dense sample of molecules is required.
Among the different types of molecules, polar molecules are of particular interest since their electric dipole moment introduces a long-range interaction between the molecules.
At the heart of our experiment is a cryogenic buffer-gas beam source which creates a high-flux beam of molecules at a few Kelvin.
Such a beam is slow enough to apply laser radiation pressure to slow the molecules below the capture velocity of a magneto-optical trap and trap them. Subsequent cooling techniques then allow for eventually reaching the ultracold regime.
AlCl Level Scheme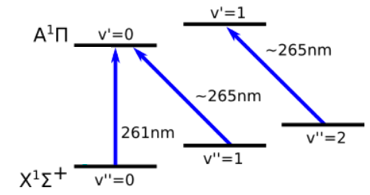
Our goal is to cool and trap aluminum monochloride (AlCl).
The theoretically predicted Franck-Condon factors of AlCl are excellent (>99%). In principle, one can scatter about 1,000 photons on AlCl before exciting the molecule vibrationally. The technically challenging part is the transition wavelength which is in the deep ultraviolet at 261nm.
UV laser system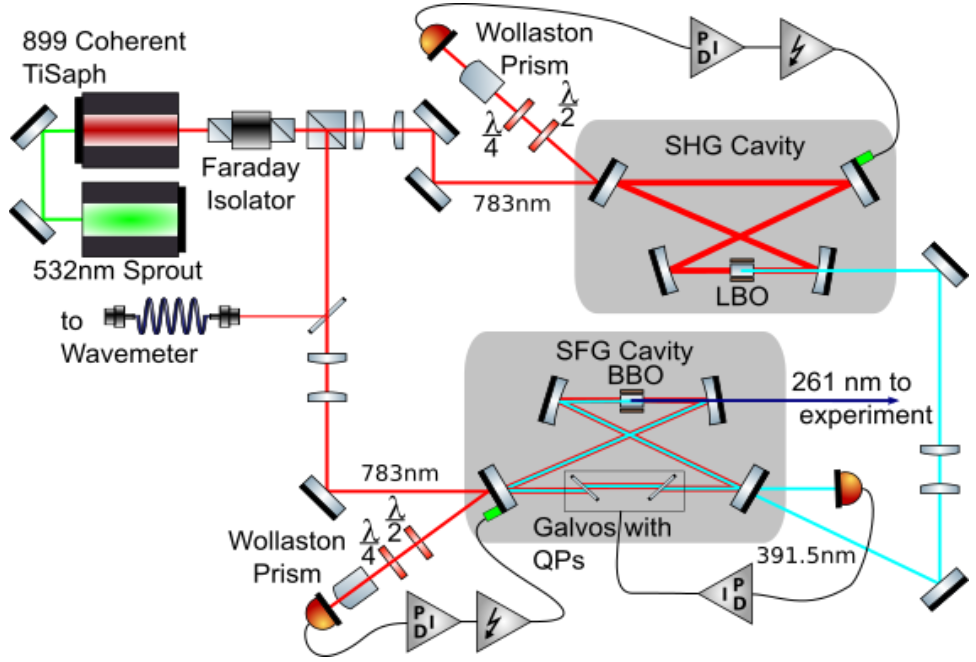
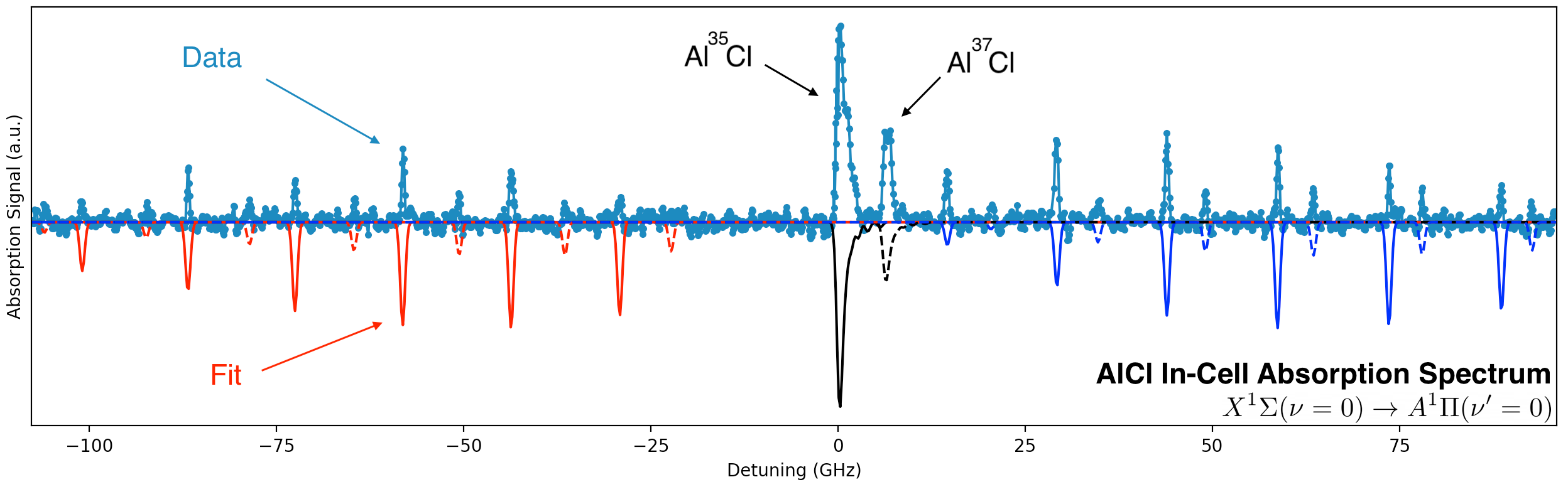
We carried out the first detailed survey spectroscopy of AlCl using our self-built UV laser system. As shown in the plot, we observe the P, Q and R rotational branches of the X-A transition and we easily resolve the isotope shift of the two chlorine isotopes.
The results of our study showed that AlCl can be produced by ablating mixtures of Al and salts (Physical Chemistry Chemical Physics 23, 22785 (2021)) and that AlCl has Franck-Condon factors of 99.88% (Physical Review A 104, 012801 (2021)).
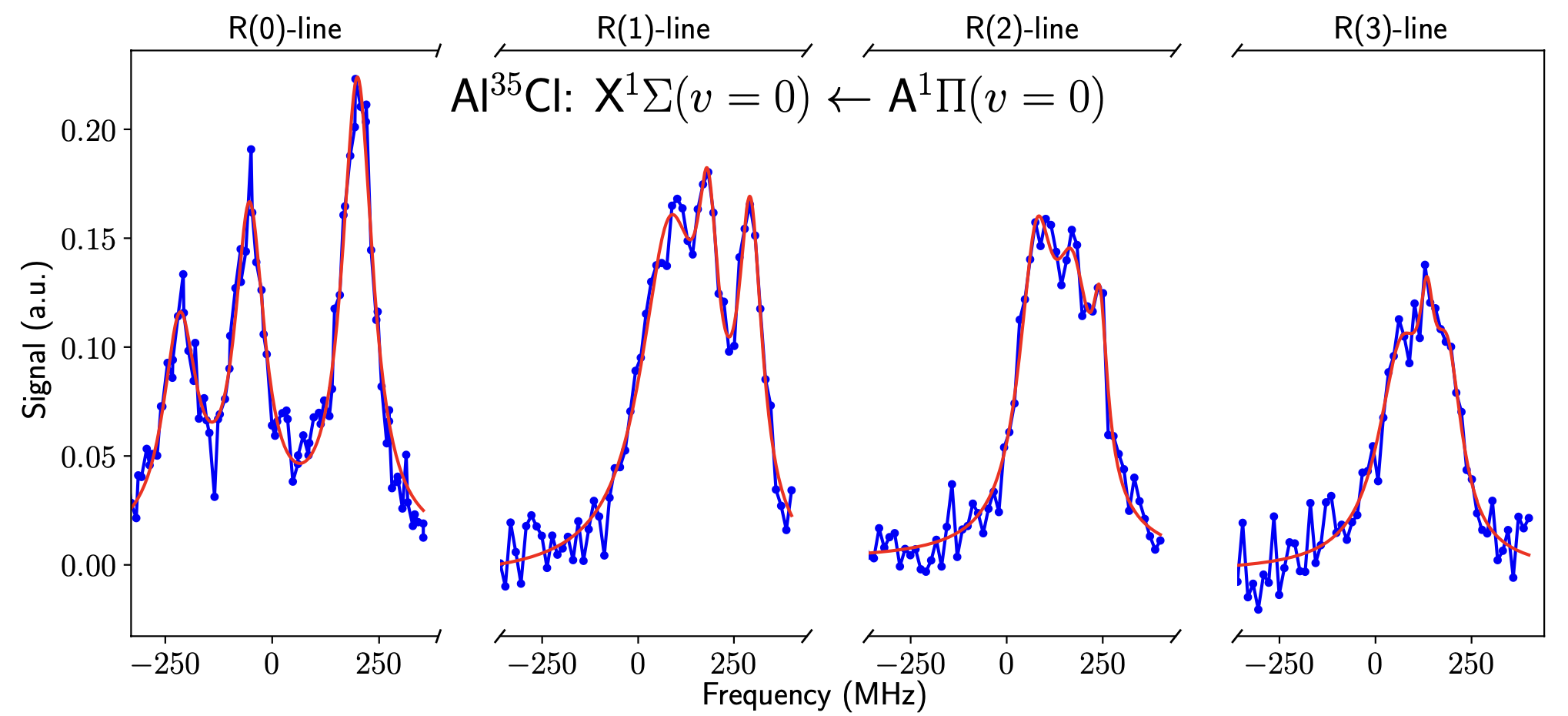
Using laser-induced fluorescence, we were able to map out the hyperfine structure of the A-X transition in AlCl, see plot below. To minimize UV-induced damage when operating the SHG cavity which produces 261.5nm light by doubling 523nm light, we operated the laser setup in a pulsed mode. For each data point, the cavity was ramped close to the resonance, at which point the cavity stabilization feedback is switched on for only approx. 50ms. With a repetition rate of about 1Hz, this method reduces the duty cycle for the UV laser to approx. 5%.
First Yb MOT @ UCR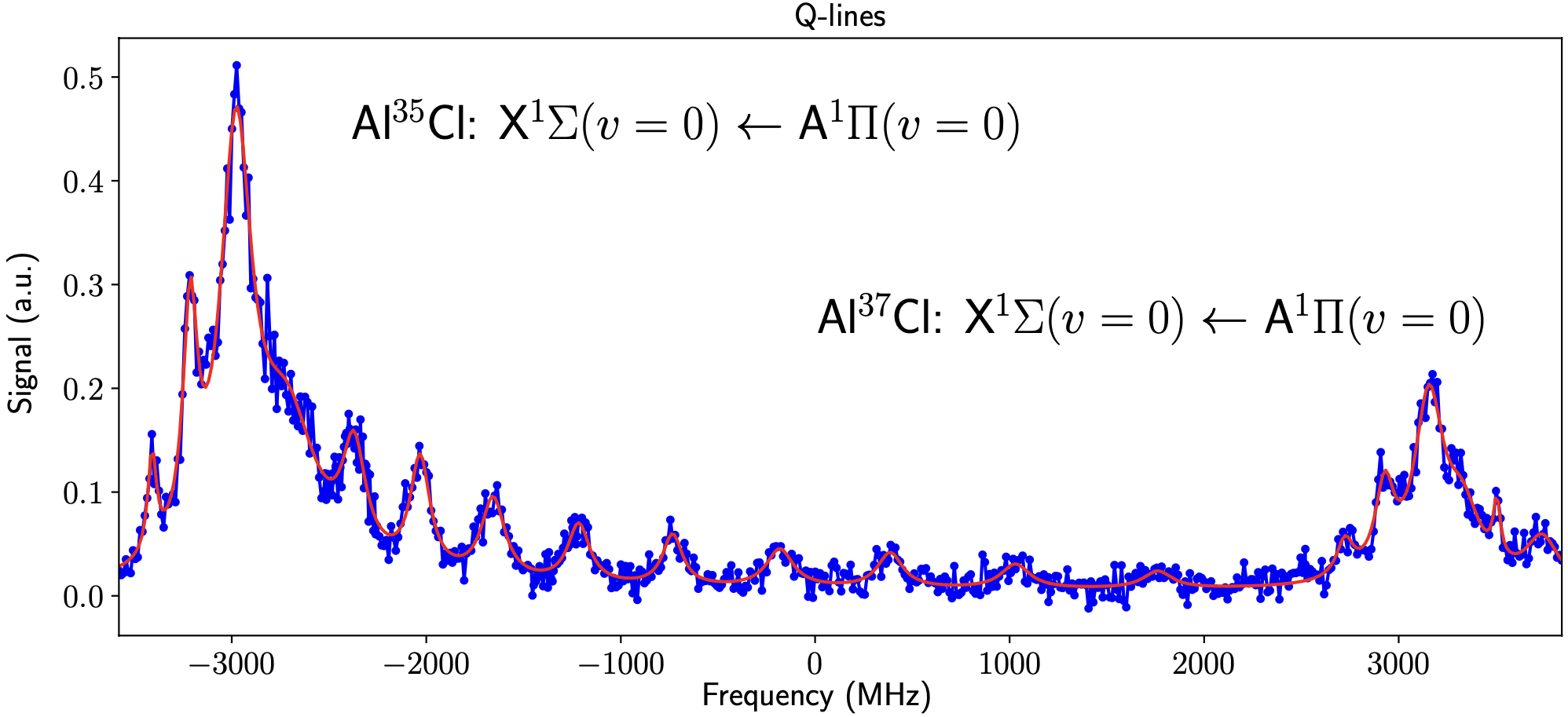
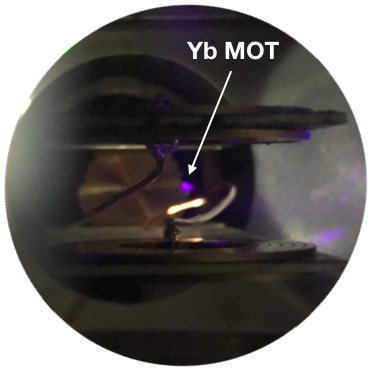
Since our laser system is very tunable, we were able to independently test our magneto-optical trap setup which is attached to the cryogenic beam source with atoms. We are happy to present our first trapped Ytterbium cloud, see photo on the right. The same tunability will allow us to explore new atomic species which have not been trapped before.
Enabling Scalable Quantum Computing
The implementation of quantum algorithms for useful applications requires the control of a large number of qubits. Trapped charged particles are excellent candidates to realize this endeavour and to build a quantum computer. In this project, we explore the possibility of creating a scalable quantum computing platform by combining the advantages of chip-based and macroscopic Paul traps. Using a two-photon polymerization direct laser writing technique (2PP-DLW), we will 3D-print miniaturized ion traps that potentially outperform their traditional counterparts.
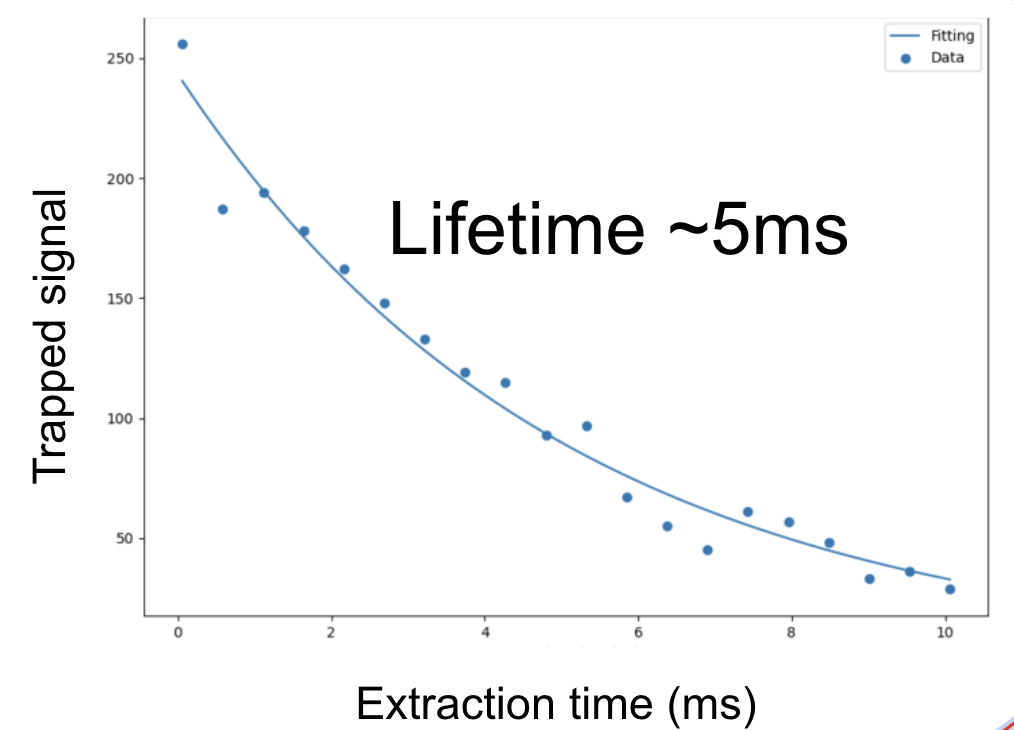
We are also exploring trapped electrons as novel carriers of quantum information. As a first step, we recently managed to trap electrons in a linear PCB Paul trap (picture on the left) and hold them for 5ms (lifetime measurement on the right).

This project is a collaboration of groups at the University of California (UCB, UCLA, UCSB, UCR).
We would like to acknowledge funding for this project through the
UC National Laboratory Fees Research Program and AFOSR.


